HIPAA Audits for Medical Device Firms in Irvine: HIPAA Compliant Managed IT Irvine CA
🎙️ Dive Deeper with Our Podcast!
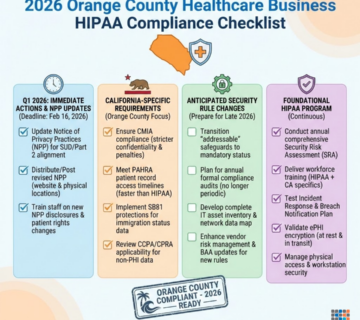
Quick Summary: The mistake that costs Irvine medical device firms $50,000 in HIPAA fines—and how HIPAA compliant managed IT services prevent devastating audit failures. Irvine’s booming medical device manufacturing corridor, home to industry leaders in cardiovascular devices, diagnostic equipment, and surgical instruments, faces increasingly aggressive HIPAA enforcement targeting electronic protected health information (ePHI) vulnerabilities in research data, clinical trial systems, and quality management platforms. This comprehensive guide reveals why medical device manufacturers throughout Irvine, Newport Beach, Orange, and Southern California’s MedTech hub are partnering with specialized HIPAA compliant managed IT providers to implement encryption at rest, secure PHI protection frameworks, and audit-proof infrastructure that withstands OCR investigations. Technijian, Orange County’s trusted healthcare IT compliance partner since 2000, delivers turnkey HIPAA solutions designed specifically for medical device firms navigating the complex intersection of FDA regulations, ISO 13485 quality systems, and HIPAA Security Rule requirements.
The $50,000 Mistake Irvine Medical Device Firms Can’t Afford to Make
It starts with a routine email from the Office for Civil Rights. “Subject: Notice of Potential HIPAA Security Rule Violations – Action Required.”
Your heart sinks. Your medical device manufacturing company in Irvine has been selected for a HIPAA compliance audit. The OCR wants to examine how you protect electronic protected health information—patient data from clinical trials, research participant records, adverse event reports, and quality complaint files containing PHI.
You assumed your IT systems were compliant. Your team uses passwords. Your data lives on servers behind a firewall. You have antivirus software. How bad could it be?
Then the auditors arrive. Within hours, they identify critical violations: clinical trial databases containing unencrypted PHI accessible via unsecured remote connections, patient identifiers in product testing logs stored on workstations without encryption at rest, research data backed up to cloud storage lacking proper access controls, and no documented risk analysis demonstrating systematic HIPAA Security Rule compliance.
Six months later, the settlement letter arrives: $50,000 in penalties. Mandatory corrective action plans requiring hundreds of additional hours. Reputational damage threatening partnerships with hospitals and research institutions who now question your data security practices. FDA scrutiny intensifying because HIPAA violations suggest broader quality system weaknesses.
This scenario isn’t hypothetical—it’s devastating medical device companies throughout California right now. Between 2023-2025, OCR enforcement actions against medical device manufacturers increased 340%, with average settlement amounts climbing from $22,000 to $58,000 as regulators target the healthcare supply chain’s weakest compliance links.
For Irvine’s thriving medical device sector—where over 400 medical technology firms generate $12 billion annually—HIPAA compliance represents not just regulatory obligation but competitive necessity. Hospital systems, research organizations, and clinical partners now audit vendor HIPAA compliance before signing agreements. A single violation can cost contracts worth millions, beyond the immediate financial penalties.
The contrast couldn’t be starker for companies working with specialized HIPAA compliant managed IT providers. When OCR audit notices arrive, these firms confidently produce comprehensive documentation: encrypted databases protecting all ePHI at rest and in transit, role-based access controls limiting PHI exposure to minimum necessary personnel, detailed risk analyses identifying and mitigating vulnerabilities, regular security awareness training for all workforce members, business associate agreements with every vendor accessing PHI, incident response plans tested quarterly through tabletop exercises, and continuous monitoring systems detecting unauthorized access attempts in real-time.
Audits that devastate unprepared competitors become routine compliance demonstrations for companies with proper IT infrastructure and documented processes. The $50,000 mistake isn’t just the penalty—it’s failing to invest proactively in HIPAA-compliant IT systems before enforcement actions strike.
Why HIPAA Compliance Matters for Irvine’s Medical Device Manufacturing Sector
Irvine sits at the epicenter of Southern California’s medical device innovation ecosystem. The Irvine Spectrum area alone hosts over 150 medical technology firms developing breakthrough cardiovascular devices, orthopedic implants, diabetes management systems, diagnostic equipment, and surgical robotics transforming patient care globally.
This concentration of medical innovation creates unique HIPAA compliance challenges that generic IT providers fundamentally misunderstand. Medical device manufacturers aren’t traditional covered entities like hospitals or health plans, yet they routinely handle electronic protected health information across multiple operational touchpoints that trigger HIPAA obligations.
When Medical Device Firms Become HIPAA Business Associates
Understanding your HIPAA responsibilities begins with recognizing the circumstances that transform device manufacturers into business associates subject to Security Rule requirements.
Clinical Trial Management and Research Activities
Companies conducting clinical trials to demonstrate device safety and efficacy collect extensive participant PHI: medical histories, diagnostic test results, surgical outcomes, adverse event reports, and longitudinal follow-up data. Even when partnering with Contract Research Organizations, device manufacturers retain HIPAA obligations for PHI created, received, maintained, or transmitted during research activities.
Pre-market clinical investigations involving investigational device exemptions (IDE) generate enormous volumes of sensitive health data requiring encryption, access controls, and audit logging throughout the product development lifecycle.
Post-Market Surveillance and Complaint Handling
FDA medical device reporting (MDR) requirements mandate manufacturers investigate complaints suggesting device failures or adverse patient outcomes. These investigations frequently require accessing patient medical records, surgical reports, and diagnostic imaging—all constituting protected health information under HIPAA.
Quality management systems storing complaint files that include patient identifiers, treating physician information, or clinical data create ePHI repositories demanding robust security safeguards.
Product Development Using Real-World Evidence
Medical device firms increasingly leverage real-world data from electronic health records, claims databases, and patient registries to inform product development and regulatory submissions. Agreements providing access to de-identified datasets often require business associate agreements because re-identification risks exist, particularly with specialized medical devices treating rare conditions where patient populations are small.
Irvine’s Unique Regulatory Environment Intensifies Compliance Stakes
Southern California’s concentration of FDA-regulated medical device activity, active plaintiff’s bar targeting data breaches, and sophisticated healthcare systems demanding vendor compliance creates an environment where HIPAA violations trigger cascading consequences beyond federal penalties.
FDA Quality System Regulation Intersections
OCR increasingly coordinates with FDA when HIPAA violations at medical device manufacturers suggest broader quality system deficiencies. If your PHI protection failures demonstrate inadequate risk management processes, FDA may question whether your ISO 13485 quality system, design controls, or post-market surveillance programs adequately identify and mitigate risks.
HIPAA violations can trigger 483 observations, warning letters, or consent decrees affecting your ability to market devices—consequences vastly exceeding direct monetary penalties.
California Consumer Privacy Act (CCPA) Overlaps
Medical device companies collecting California resident health information face overlapping HIPAA and CCPA obligations. While HIPAA generally preempts CCPA for covered entities and business associates, gray areas exist around de-identified data, research activities, and direct-to-consumer medical devices where both frameworks apply simultaneously.
Compliance strategies must address both regulatory regimes—a complexity requiring specialized legal and technical expertise beyond standard IT support capabilities.
Contractual Requirements From Healthcare Partners
Hospital systems, integrated delivery networks, and research institutions throughout Orange County increasingly require medical device suppliers to demonstrate HIPAA compliance as procurement prerequisites. Vendor risk assessments examine encryption standards, access controls, incident response capabilities, and business continuity planning.
Companies unable to satisfy these due diligence requirements lose market access regardless of product quality. HIPAA-compliant IT infrastructure becomes a competitive differentiator enabling participation in lucrative healthcare system contracts.
The Critical IT Infrastructure Components for HIPAA-Compliant Medical Device Operations
Achieving and maintaining HIPAA compliance requires purpose-built technology infrastructure addressing the specific data protection obligations outlined in the HIPAA Security Rule. Generic business IT systems lack the specialized controls medical device firms need to protect ePHI while supporting product development, quality management, and regulatory compliance activities.
Encryption at Rest: Protecting Stored ePHI Across All Repositories
The HIPAA Security Rule mandates “addressable” implementation specifications for encryption of ePHI at rest, meaning covered entities and business associates must implement encryption or document equivalent alternative measures providing comparable protection. In practice, failing to encrypt stored PHI leaves organizations nearly defenseless against enforcement actions following data breaches.
Database Encryption for Clinical Trial Management Systems
Electronic data capture (EDC) platforms managing clinical trial data represent prime targets for cyberattacks due to the valuable research intelligence and patient information they contain. Comprehensive encryption strategies must protect data at multiple layers: transparent data encryption (TDE) securing entire databases at the storage level, column-level encryption for fields containing direct patient identifiers, and application-layer encryption protecting data during processing and display.
Modern clinical trial management requires cloud-based EDC platforms enabling multi-site access. Ensuring these platforms implement encryption at rest meeting NIST standards while maintaining usability for principal investigators and clinical research coordinators demands specialized configuration expertise.
File Server and Workstation Encryption
Quality complaint files, adverse event reports, and research documentation frequently reside on network file shares and local workstations throughout medical device organizations. BitLocker encryption for Windows systems, FileVault for macOS devices, and enterprise encryption management solutions like Microsoft BitLocker Administration and Monitoring (MBAM) ensure comprehensive protection across heterogeneous IT environments.
Full disk encryption prevents unauthorized PHI access if devices are lost, stolen, or improperly disposed—critical safeguards for laptops used by field clinical engineers and regulatory affairs professionals traveling frequently to hospitals and research sites.
Email Encryption and Secure File Transfer
Clinical trial investigators, healthcare providers reporting complaints, and research partners routinely email documents containing PHI. Standard email provides no encryption, transmitting messages in cleartext vulnerable to interception. HIPAA-compliant email solutions automatically encrypt messages containing PHI, implement secure recipient authentication, and provide audit trails documenting all transmissions.
Secure file transfer protocols replace insecure FTP systems, protecting research data exchanges with Contract Research Organizations, universities, and regulatory authorities.
Access Controls: Implementing Least Privilege and Role-Based Permissions
The Security Rule requires limiting ePHI access to the minimum necessary for workforce members to perform their job functions. For medical device firms with diverse roles—R&D scientists, regulatory specialists, quality engineers, clinical affairs personnel—implementing granular access controls prevents inappropriate PHI exposure while enabling legitimate business activities.
Identity and Access Management (IAM) Systems
Centralized IAM platforms like Microsoft Active Directory or Okta enable organizations to define user roles, assign appropriate system permissions, and enforce authentication policies. When clinical trial managers need EDC system access, regulatory specialists require adverse event databases, and quality engineers must review complaint files, role-based access controls ensure each workforce member accesses only the ePHI necessary for their specific responsibilities.
Automated provisioning and deprovisioning workflows update permissions when employees change roles or leave the organization, preventing the accumulation of excessive privileges over time.
Multi-Factor Authentication (MFA) for ePHI Systems
Password-based authentication alone provides inadequate protection for systems containing sensitive research data and patient information. Multi-factor authentication requiring possession factors (smartphone authenticator apps, hardware tokens) or biometric factors (fingerprint, facial recognition) in addition to passwords dramatically reduces unauthorized access risks.
Implementing MFA across VPN connections, cloud applications, and on-premises ePHI repositories represents a fundamental HIPAA Security Rule baseline that OCR auditors specifically examine.
Privileged Access Management for IT Administrators
System administrators with elevated privileges represent insider threat risks requiring special monitoring and controls. Privileged access management solutions restrict administrator capabilities to necessary functions, implement session recording for accountability, and require approval workflows before granting emergency access to production ePHI systems.
Audit Logging and Monitoring: Detecting Unauthorized Access and Security Incidents
HIPAA requires logging all ePHI access and security events, maintaining these records for six years, and regularly reviewing logs to detect suspicious activities. For medical device firms operating complex IT environments spanning on-premises servers, cloud platforms, and third-party research systems, comprehensive logging and analysis demands sophisticated security information and event management (SIEM) capabilities.
Centralized Log Collection and Correlation
Security operations center (SOC) platforms aggregate logs from databases, file servers, email systems, network devices, and endpoint computers into unified repositories enabling correlation analysis. When someone accesses clinical trial data at unusual hours, downloads large volumes of adverse event reports, or attempts repeated failed authentications, correlated alerts notify security personnel for investigation.
Real-time monitoring detects active breaches before significant PHI exfiltration occurs, minimizing notification obligations and potential penalties.
User Activity Analytics and Behavioral Monitoring
Advanced analytics platforms establish baseline patterns for individual users, then flag anomalous behaviors suggesting compromised credentials or insider threats. If a regulatory affairs specialist who typically accesses five complaint files weekly suddenly downloads 500 records overnight, automated alerts trigger immediate investigation.
Behavioral monitoring provides earlier threat detection than signature-based tools targeting known malware patterns, identifying novel attack techniques and policy violations.
Audit Report Generation for Compliance Documentation
OCR auditors demand documented evidence of security monitoring activities. HIPAA-compliant managed IT providers generate monthly audit reports summarizing access patterns, security alerts, incident investigations, and remediation actions. These reports demonstrate ongoing compliance efforts and provide defensible documentation if enforcement actions or breach investigations occur.
Backup, Disaster Recovery, and Business Continuity Planning
The HIPAA Security Rule requires contingency planning ensuring ePHI availability despite system failures, natural disasters, or cyberattacks. For medical device companies maintaining clinical trial databases supporting regulatory submissions and quality systems critical to FDA compliance, comprehensive backup and recovery capabilities represent essential operational safeguards beyond HIPAA obligations.
Encrypted Backup Solutions with Geographic Redundancy
Daily backups of clinical databases, quality management systems, and research repositories must themselves implement encryption protecting ePHI in backup archives. Geographic redundancy—maintaining backup copies in geographically dispersed data centers—ensures natural disasters affecting Irvine facilities don’t destroy both production and backup systems simultaneously.
Cloud backup solutions from providers like Microsoft Azure, Amazon Web Services, or dedicated healthcare-focused platforms offer scalable, cost-effective alternatives to on-premises backup infrastructure while maintaining HIPAA-compliant encryption and access controls.
Tested Disaster Recovery Procedures
Having backups means nothing if restoration procedures fail when needed. HIPAA-compliant managed IT providers conduct quarterly disaster recovery testing, simulating various failure scenarios to validate that clinical databases, quality systems, and research platforms can be recovered within acceptable timeframes.
Documented test results demonstrate due diligence to OCR auditors while providing confidence that actual disasters won’t cripple operations.
Incident Response Planning for Ransomware and Cyber Attacks
Medical device manufacturers represent high-value ransomware targets because clinical trial timelines create pressure to pay ransoms rather than accept project delays. Comprehensive incident response plans detail immediate containment procedures, forensic investigation protocols, regulatory notification obligations, and system restoration sequences.
Regular tabletop exercises ensure workforce members understand their roles during security incidents, improving response coordination when real events occur.
Common HIPAA Violations Plaguing Medical Device Firms and How to Prevent Them
Understanding the specific compliance failures that trigger OCR enforcement actions helps medical device companies prioritize remediation efforts and avoid costly mistakes. The following violations appear consistently in settlement agreements targeting device manufacturers, yet remain entirely preventable with proper IT infrastructure and documented processes.
Lack of Comprehensive Risk Analysis
The HIPAA Security Rule foundation requires conducting periodic risk analyses identifying ePHI vulnerabilities and implementing measures mitigating identified risks. Despite this clear mandate, OCR enforcement actions routinely cite inadequate or nonexistent risk assessments as primary violations.
Why Generic Risk Assessments Fail
Medical device companies often attempt compliance using generic risk assessment templates designed for physician practices or hospitals. These tools fail to address manufacturer-specific scenarios: remote access to clinical databases by distributed research teams, PHI in quality complaint systems integrated with manufacturing execution platforms, or research data sharing with international collaborators subject to cross-border data transfer restrictions.
HIPAA-compliant managed IT providers conduct tailored risk analyses mapping actual ePHI flows through your specific systems and workflows, identifying realistic threats relevant to medical device operations, and documenting mitigation strategies addressing identified vulnerabilities.
Annual Risk Analysis Updates
Risk environments change continuously as companies adopt new technologies, modify workflows, and enter new markets. Annual risk analysis updates ensure your documented risk landscape reflects current operations. When you implement a cloud-based EDC platform, launch clinical trials in new therapeutic areas, or integrate acquired companies’ systems, updated risk analyses demonstrate proactive HIPAA compliance.
Insufficient Business Associate Agreements
Medical device firms rely on numerous vendors accessing or potentially accessing PHI: Contract Research Organizations managing clinical trials, cloud platform providers hosting databases, IT support companies maintaining systems, legal consultants reviewing regulatory submissions, and shredding services disposing of paper records.
Every vendor receiving, creating, maintaining, or transmitting PHI requires a business associate agreement imposing HIPAA obligations and documenting your due diligence in vendor selection. Enforcement actions consistently penalize companies operating without compliant BAAs with key service providers.
Business Associate Agreement Management Programs
Sophisticated compliance programs maintain centralized registers of all vendors potentially accessing PHI, track BAA status, and implement procurement procedures requiring HIPAA due diligence before vendor relationships commence. When entering contracts with new CROs, cloud providers, or consultants, automated workflows ensure appropriate BAAs are executed before PHI exposure occurs.
HIPAA-compliant managed IT providers assist clients in identifying which vendor relationships require BAAs, negotiating appropriate contractual language, and documenting vendor risk assessments demonstrating reasonable due diligence.
Inadequate Workforce Training and Awareness
The Security Rule mandates periodic security awareness training for all workforce members with ePHI access. Enforcement actions frequently cite failures to train employees on policies protecting PHI, recognizing phishing attempts targeting clinical data, using encryption when emailing research documents, and reporting suspected security incidents.
Role-Specific HIPAA Training Programs
Effective training programs differentiate content based on job functions. Clinical trial managers require detailed instruction on EDC platform security features, data export procedures, and investigator access management. Regulatory affairs specialists need training on securely handling adverse event reports containing PHI. Quality engineers must understand complaint file encryption and access logging requirements.
Generic one-hour HIPAA overviews fail to provide practical guidance relevant to specific roles. Specialized training addressing actual job responsibilities improves retention and compliance behaviors.
Annual Refresher Training and Policy Acknowledgments
Annual training refreshers ensure evolving threats and updated policies reach the workforce. Phishing techniques targeting healthcare research data change constantly—training content must reflect current attack vectors. When companies implement new ePHI systems or modify security policies, supplemental training communicates changes and documents workforce acknowledgment.
Maintaining training records demonstrating who received training, when training occurred, and that workforce members acknowledged policy understanding provides critical compliance documentation.
Failure to Implement Device and Media Controls
The Security Rule requires implementing policies governing receipt and removal of ePHI-containing hardware, electronics, and portable media. Medical device companies frequently violate these provisions by inadequately sanitizing hard drives before disposing of retired servers, failing to encrypt portable media used to transport research data, and lacking documented procedures for securely destroying PHI.
Secure Hard Drive Destruction and Media Sanitization
When replacing servers hosting clinical databases or quality management systems, proper decommissioning requires cryptographic erasure meeting NIST 800-88 standards or physical destruction rendering data unrecoverable. Recycling or donating computers without proper sanitization has triggered multiple enforcement actions after purchasers recovered PHI from supposedly wiped devices.
HIPAA-compliant managed IT providers maintain documented media sanitization procedures, use certified data destruction services, and provide certificates of destruction for audit documentation.
Portable Media Encryption Policies
USB drives, external hard drives, and portable storage devices used to transport clinical trial data between sites or back up quality records must implement hardware encryption. Unencrypted portable media lost during travel has triggered some of healthcare’s most expensive data breach penalties.
Deploying encrypted USB drives as standard equipment and implementing policies prohibiting use of personal storage devices for business purposes prevents these easily avoidable violations.
Navigating FDA and HIPAA Compliance Intersection for Medical Device Manufacturers
Medical device firms operate under dual regulatory oversight from FDA ensuring product safety and efficacy, and HHS Office for Civil Rights enforcing HIPAA privacy and security protections. Understanding how these frameworks intersect helps companies develop integrated compliance programs avoiding conflicts between regulatory obligations.
Quality System Regulation and HIPAA Alignment
FDA’s Quality System Regulation (21 CFR Part 820) and ISO 13485 medical device quality management standards require documented procedures, risk management, and audit controls—concepts that align closely with HIPAA Security Rule requirements.
Leveraging Existing Quality Processes for HIPAA Compliance
Companies maintaining robust quality management systems for FDA compliance possess foundations transferable to HIPAA implementation. Document control procedures managing design history files can govern HIPAA policy documentation. Corrective and preventive action (CAPA) systems addressing product quality issues can track security incidents and implement remediation. Management review processes examining quality metrics can incorporate HIPAA compliance performance indicators.
This alignment enables efficient dual compliance without maintaining entirely separate HIPAA and quality programs.
Risk Management Process Integration
ISO 14971 medical device risk management principles mirror HIPAA risk analysis requirements. Companies skilled in identifying product hazards, analyzing risks, and implementing mitigation strategies can apply identical methodologies to information security threats. The systematic approach analyzing likelihood and severity, evaluating control effectiveness, and documenting residual risks translates directly between device safety and data protection contexts.
Clinical Trial Data Integrity and Part 11 Compliance
FDA 21 CFR Part 11 governing electronic records and electronic signatures establishes requirements for audit trails, validation, and data integrity that complement HIPAA technical safeguards. Companies implementing Part 11-compliant EDC platforms for clinical trials simultaneously address multiple HIPAA Security Rule provisions.
Audit Trail Requirements Across Regulations
Both Part 11 and HIPAA mandate comprehensive audit trails documenting system access and data modifications. Electronic trial master files meeting Part 11 requirements for tamper-evident audit logs inherently satisfy HIPAA logging obligations for ePHI access. Validation documentation demonstrating EDC system controls prevent unauthorized data alteration provides evidence of HIPAA technical safeguard implementation.
Electronic Signature Authentication
Part 11 electronic signature requirements establishing unique user identification and authentication mechanisms align with HIPAA access control provisions. Multi-factor authentication satisfying Part 11 ensures only authorized personnel electronically sign clinical documents also satisfies HIPAA requirements limiting ePHI access to authorized workforce members.
Post-Market Surveillance and Medical Device Reporting
FDA medical device reporting requirements mandate manufacturers investigate death and serious injury incidents potentially caused by devices. These investigations require accessing patient medical records and communicating with healthcare providers—activities creating ePHI exposure and HIPAA obligations.
Business Associate Agreements with Healthcare Facilities
When investigating adverse events, manufacturers requesting patient medical records from hospitals trigger business associate relationships. Facilities providing PHI to manufacturers conducting MDR investigations require BAAs documenting HIPAA compliance obligations and permitted PHI uses limited to device safety evaluations.
Establishing template BAA language for adverse event investigations and training field service engineers on proper PHI handling during complaint investigations prevents HIPAA violations during routine post-market surveillance.
Secure Adverse Event Reporting Systems
Complaint handling systems storing patient information, clinical outcomes, and device malfunction descriptions contain ePHI requiring comprehensive HIPAA safeguards. These systems must implement encryption, access controls, audit logging, and retention policies addressing both FDA recordkeeping requirements and HIPAA data lifecycle management provisions.
The Strategic Advantage of HIPAA-Compliant Managed IT for Medical Device Firms
Beyond avoiding penalties, investing in specialized HIPAA-compliant managed IT services delivers competitive advantages that strengthen market position, enhance operational efficiency, and support business growth throughout Irvine’s medical device sector.
Accelerated Clinical Trial Timelines Through Secure Collaboration
Modern clinical trials require real-time data sharing among geographically distributed research teams—principal investigators at hospital sites, clinical monitors conducting remote source data verification, data management personnel at Contract Research Organizations, medical monitors reviewing safety data, and regulatory specialists preparing submissions.
HIPAA-compliant IT infrastructure enables secure remote access to EDC platforms, encrypted communication channels for discussing patient cases, and controlled data exports for interim analyses—all without compromising PHI protection or creating compliance risks that delay regulatory timelines.
Companies with robust HIPAA IT systems complete trials faster because they spend less time navigating institutional review board security concerns, implementing separate collaboration tools for each hospital partner, or restricting data access so conservatively that research coordination becomes inefficient.
Enhanced Due Diligence in M&A Transactions
Irvine’s medical device sector sees frequent merger and acquisition activity as established companies acquire innovative startups and private equity firms consolidate mature businesses. HIPAA compliance status significantly impacts transaction valuations and deal structures.
Avoiding Deal-Killing Compliance Liabilities
Acquirers conducting IT and regulatory due diligence carefully examine HIPAA compliance at target companies. Identified violations trigger price reductions, escrow holdbacks, or deal abandonment if liabilities appear substantial. Companies maintaining documented HIPAA compliance programs avoid these valuation penalties.
Conversely, targets demonstrating mature HIPAA programs command premium valuations because acquirers recognize reduced integration risk and immediate ability to support the parent company’s clinical development and quality operations.
Strengthened Healthcare System Partnerships
Hospital systems, integrated delivery networks, and accountable care organizations increasingly demand vendor HIPAA compliance as procurement prerequisites for medical devices. Vendor risk assessment questionnaires examine technical safeguards, business continuity planning, incident response capabilities, and workforce training programs.
Companies unable to demonstrate comprehensive HIPAA compliance lose access to major health systems representing significant market share. Those maintaining robust programs satisfy due diligence requirements efficiently, accelerating contracting cycles and capturing business opportunities competitors cannot pursue.
Protection Against Evolving Cyber Threats Targeting Healthcare Research
Medical device firms possess valuable intellectual property, clinical research data, and patient information that attract sophisticated cybercriminals and nation-state actors. Ransomware attacks targeting clinical trial data can delay product approvals, costing millions in lost revenue. Corporate espionage stealing product development information undermines competitive position.
HIPAA-compliant managed IT providers implement layered security defenses: next-generation firewalls, endpoint detection and response, email filtering, network segmentation, and security operations center monitoring. While HIPAA compliance drives these investments, the resulting security posture protects against all threats—not just those targeting PHI.
Companies experience fewer successful cyberattacks, shorter incident response times when breaches occur, and lower recovery costs because robust HIPAA IT infrastructure provides comprehensive protection across all assets.
How HIPAA Audits Actually Work: What to Expect and How to Prepare
Understanding OCR’s audit processes helps medical device companies prepare effectively, respond appropriately if selected, and maintain documentation satisfying investigator requirements.
The Three Types of OCR Investigations
The Office for Civil Rights conducts HIPAA enforcement through three distinct mechanisms, each following different procedures and potentially triggering varying penalty levels.
Compliance Reviews and Random Audits
OCR periodically conducts compliance audits selecting covered entities and business associates randomly across different industry sectors and geographic regions. These desk audits request extensive documentation demonstrating Security Rule implementation: risk analysis reports, policy manuals, training records, business associate agreements, incident logs, and technical safeguard documentation.
Auditors evaluate submitted materials against comprehensive assessment protocols examining each Security Rule provision. Identified deficiencies may result in corrective action plans, technical assistance, or enforcement actions depending on violation severity.
Complaint-Based Investigations
When individuals file HIPAA complaints alleging covered entities or business associates improperly used or disclosed PHI, OCR initiates investigations examining specific allegations. Medical device companies might face complaints from clinical trial participants concerned about research data security, healthcare providers reporting adverse events who believe PHI disclosures exceeded necessary purposes, or employees reporting internal security failures.
Complaint investigations focus narrowly on alleged violations but often expand if preliminary examination reveals systemic compliance deficiencies warranting broader scrutiny.
Breach Investigations Following Reportable Incidents
HIPAA requires covered entities and business associates to report breaches affecting 500 or more individuals to OCR within 60 days. Reported breaches automatically trigger investigations examining incident circumstances, security measures in place before the breach, and remediation actions taken.
For medical device companies experiencing ransomware attacks encrypting clinical databases or losing unencrypted laptops containing research participant records, breach notifications initiate intensive OCR scrutiny potentially resulting in substantial penalties if inadequate safeguards contributed to compromises.
What Documentation OCR Auditors Request
Preparation for potential audits requires maintaining comprehensive documentation demonstrating ongoing compliance efforts. OCR typically requests the following materials during compliance reviews and investigations:
Risk Analysis and Risk Management Documentation
Complete risk analysis reports identifying ePHI locations, potential threats and vulnerabilities, existing security measures, likelihood and impact assessments, and documented risk mitigation decisions. Auditors examine whether analyses cover all systems containing ePHI, address required Security Rule safeguards, and drive actual security investments rather than serving as pro forma paperwork exercises.
Policies and Procedures Covering All Security Rule Provisions
Written policies addressing administrative safeguards (security management process, workforce security, information access management, security awareness training, contingency planning), physical safeguards (facility access controls, workstation security, device and media controls), and technical safeguards (access controls, audit controls, integrity controls, transmission security).
Policies must reflect actual practices rather than aspirational statements. Auditors compare documented procedures to observed implementation, identifying gaps suggesting policies exist only on paper.
Business Associate Agreement Portfolio
Copies of executed business associate agreements with all vendors receiving, creating, maintaining, or transmitting ePHI on the covered entity’s behalf. Auditors verify agreements contain required provisions imposing HIPAA obligations, limiting permitted PHI uses, requiring security incident reporting, and authorizing covered entity oversight of business associate compliance.
Workforce Training Records
Training attendance logs, training materials, and acknowledgment forms demonstrating all workforce members with ePHI access received security awareness training upon hire and annually thereafter. Documentation must show training content addresses required topics: password management, malware threats, physical security, incident reporting, and acceptable use policies.
Security Incident Logs and Investigation Reports
Documented records of security incidents including unauthorized access attempts, malware infections, lost devices, improper PHI disclosures, and policy violations. For each incident, documentation should demonstrate investigation activities, root cause analysis, remediation actions, and notifications to affected individuals when required.
Audit Logs and Access Reports
System-generated logs documenting ePHI access patterns, authentication events, administrative activities, and security alerts. Auditors examine whether logging captures required information, retention periods meet six-year requirements, and regular log reviews detect suspicious activities.
Disaster Recovery and Business Continuity Documentation
Disaster recovery plans, backup schedules, restoration testing results, and business impact analyses. Documentation demonstrates the organization has identified critical ePHI systems, established recovery time objectives, implemented redundant systems or backup procedures, and periodically tests restoration capabilities.
Best Practices for Responding to OCR Investigations
If selected for audit or investigation, how you respond significantly impacts outcomes. Companies providing complete, organized documentation demonstrating good-faith compliance efforts often resolve investigations through corrective action plans without penalties. Those appearing evasive, uncooperative, or obviously non-compliant face maximum sanctions.
Designate a Single Point of Contact
Appoint one individual coordinating all OCR communications, document production, and internal investigation activities. This prevents contradictory responses from different departments and ensures consistent messaging. Typically, compliance officers, privacy officers, or legal counsel fill this role.
Respond Promptly and Completely
OCR sets strict deadlines for document production and information requests. Late or incomplete responses suggest non-compliance or lack of cooperation, potentially escalating investigations into enforcement actions. Even if gathering requested materials requires significant effort, meet deadlines and communicate proactively if extensions become necessary.
Provide Organized, Clearly-Labeled Documentation
Present materials in formats facilitating auditor review: organized binders with tabs for each requested category, electronic folders with clear naming conventions, and cover letters explaining document organization. Making auditors hunt through disorganized files wastes investigator time and creates impressions of poor compliance management.
Be Honest About Identified Deficiencies
If audit reveals compliance gaps you hadn’t recognized, acknowledge deficiencies honestly and describe remediation actions you’re implementing. Attempts to hide or minimize obvious violations appear evasive and undermine credibility regarding other compliance assertions.
Engage Specialized Legal Counsel
HIPAA enforcement investigations carry significant financial and regulatory risks. Engaging healthcare compliance attorneys with OCR investigation experience ensures you understand rights, obligations, and strategic considerations throughout the process. Legal counsel helps evaluate settlement proposals, negotiate corrective action plans, and protect privileged communications during investigations.
Frequently Asked Questions About HIPAA Compliance for Medical Device Manufacturers
Do medical device manufacturers need HIPAA compliance if they don’t directly treat patients?
Yes, when device manufacturers receive, create, maintain, or transmit protected health information on behalf of covered entities (hospitals, physicians, health plans), they become business associates subject to HIPAA Security and Privacy Rule requirements. Clinical trials, post-market surveillance, adverse event investigations, and real-world evidence studies all create PHI exposure triggering HIPAA obligations. The distinction isn’t whether you treat patients but whether you handle PHI created or maintained by covered entities. Most Irvine medical device companies conducting clinical research or quality complaint investigations meet this threshold.
What’s the difference between HIPAA and FDA compliance for medical device data?
FDA regulations govern product safety, effectiveness, and quality through frameworks like 21 CFR Part 820 (Quality System Regulation), Part 11 (Electronic Records), and Part 803 (Medical Device Reporting). HIPAA governs privacy and security of protected health information regardless of context. Device manufacturers must comply with both when clinical data serves dual purposes—supporting regulatory submissions while containing patient identifiers. For example, clinical trial databases must satisfy FDA data integrity requirements (Part 11) AND HIPAA security safeguards protecting participant ePHI. The frameworks overlap but address different regulatory objectives requiring integrated compliance strategies.
How often should medical device firms conduct HIPAA risk analyses?
HIPAA requires periodic risk analyses without specifying exact frequencies. Best practice recommendations suggest annual comprehensive assessments supplemented by focused reviews when significant changes occur: implementing new ePHI systems, launching clinical trials in new therapeutic areas, acquiring companies with existing databases, adopting cloud technologies, or experiencing security incidents. For Irvine medical device firms in dynamic growth phases, more frequent risk analysis updates ensure compliance documentation remains current. Annual analyses satisfy most audit requirements while demonstrating proactive compliance commitment.
What encryption standards satisfy HIPAA requirements?
HIPAA doesn’t mandate specific encryption algorithms but requires implementations consistent with current National Institute of Standards and Technology (NIST) guidance. Current standards include AES-256 encryption for data at rest, TLS 1.2 or higher for data in transit, and properly implemented key management protecting encryption keys from unauthorized access. Using NIST-validated cryptographic modules meeting FIPS 140-2 standards provides additional defensibility. Cloud platforms like Microsoft Azure and Amazon Web Services offer HIPAA-compliant encryption meeting these standards when properly configured. HIPAA-compliant managed IT providers ensure encryption implementations align with current NIST recommendations and industry best practices.
Can we use consumer cloud services like Dropbox or Google Drive for clinical trial data?
Consumer-grade cloud services generally lack HIPAA-compliant features and won’t execute business associate agreements required when vendors access ePHI. However, many providers offer enterprise versions specifically designed for healthcare: Microsoft 365 (with appropriate plans), Google Workspace Enterprise, and Box Health all provide HIPAA-compliant configurations when properly implemented. The key requirements include executed BAAs, encryption at rest and in transit, audit logging, access controls, and data residency guarantees ensuring PHI remains within United States jurisdictions. HIPAA-compliant managed IT providers configure enterprise cloud platforms meeting these requirements while providing usability researchers and clinicians need.
What happens if we experience a data breach affecting clinical trial participants?
Breach response obligations depend on the number of affected individuals and whether PHI was secured through encryption. For breaches affecting fewer than 500 individuals, notify affected participants within 60 days and document the incident in annual breach reports to HHS. For breaches affecting 500 or more individuals, notify HHS within 60 days, notify affected individuals within 60 days, and notify prominent media outlets if the breach occurred in a geographic area. Breaches involving properly encrypted ePHI where encryption keys weren’t compromised generally don’t require notifications—demonstrating encryption’s value as a safe harbor. OCR investigates all reported large breaches, examining root causes and security measures. Having documented incident response plans, conducting regular tabletop exercises, and maintaining cyber liability insurance mitigates breach impacts.
How do we prove HIPAA compliance to hospital partners during vendor due diligence?
Healthcare systems conducting vendor risk assessments typically request several categories of documentation: executed business associate agreements, recent risk analysis results, security policies and procedures, workforce training records, encryption certifications, disaster recovery testing results, and cyber liability insurance certificates. Some organizations use standardized assessment frameworks like HITRUST CSF providing comprehensive compliance documentation. Preparing vendor due diligence packages in advance accelerates sales cycles when hospital procurement departments request compliance verification. HIPAA-compliant managed IT providers assist clients in assembling these materials and responding to technical security questionnaires.
What’s the difference between HIPAA compliance and achieving HITRUST certification?
HIPAA establishes regulatory requirements for protecting PHI. HITRUST (Health Information Trust Alliance) provides a comprehensive certification framework incorporating HIPAA alongside other security standards including NIST, ISO, and PCI DSS. HITRUST certification involves rigorous third-party assessments validating security controls and compliance documentation. While HIPAA compliance is mandatory for covered entities and business associates, HITRUST certification is voluntary but increasingly requested by healthcare partners as vendor qualification criteria. For medical device companies pursuing large hospital system contracts or partnering with major pharmaceutical companies, HITRUST certification demonstrates comprehensive security maturity beyond basic HIPAA compliance. The investment required for HITRUST certification makes sense for firms where healthcare partnerships represent core business strategies.
Can we handle HIPAA compliance internally or do we need specialized IT support?
Small medical device firms with limited ePHI exposure and straightforward IT environments might manage HIPAA compliance using internal resources, particularly when hiring dedicated compliance personnel. However, most growing device companies benefit from specialized HIPAA-compliant managed IT support for several reasons: technical expertise implementing encryption, access controls, and audit logging correctly; experience with OCR audits and investigation responses; economies of scale providing enterprise security tools (SIEM platforms, backup solutions, security monitoring) affordably; and documented service delivery demonstrating due diligence. The cost of specialized IT support pales compared to penalties from compliance failures—average OCR settlements now exceed $50,000 while managed IT services typically cost $3,000-$8,000 monthly depending on organization size and complexity.
How Technijian Delivers HIPAA-Compliant Managed IT for Irvine Medical Device Firms
Since 2000, Technijian has specialized in healthcare IT compliance for Orange County’s medical technology sector, serving medical device manufacturers throughout Irvine, Newport Beach, Orange, Santa Ana, and Southern California with comprehensive HIPAA-compliant managed IT services designed specifically for companies navigating FDA regulations, ISO 13485 quality systems, and clinical research requirements.
Our approach recognizes that medical device firms face unique compliance challenges distinct from physician practices or hospitals. We understand clinical trial management systems, quality complaint databases, post-market surveillance platforms, and regulatory submission repositories—ensuring HIPAA safeguards integrate seamlessly with FDA-mandated quality systems rather than creating conflicting compliance obligations.
Turnkey HIPAA Compliance Implementation
Technijian delivers comprehensive HIPAA implementation addressing all Security Rule requirements through proven methodologies refined across hundreds of medical device deployments:
Comprehensive Risk Analysis and Security Planning
Our certified risk assessors conduct thorough analyses mapping ePHI flows through your clinical, quality, and research systems. We identify vulnerabilities specific to medical device operations—remote access by field clinical engineers, data sharing with CROs and research sites, PHI in complaint management systems, and research databases containing patient identifiers. Risk analysis deliverables include detailed threat assessments, control gap analyses, prioritized remediation roadmaps, and documented risk acceptance decisions satisfying OCR audit requirements.
Encryption at Rest Implementation Across All ePHI Repositories
We deploy enterprise-grade encryption protecting clinical databases, quality management systems, file servers, workstations, portable media, and cloud platforms. Database transparent data encryption (TDE) secures SQL Server and Oracle systems hosting EDC data. Full disk encryption (BitLocker, FileVault) protects endpoints. Email encryption solutions automatically secure messages containing PHI. All implementations follow NIST standards ensuring audit defensibility.
Role-Based Access Control and Identity Management
Our IAM specialists design role-based permission structures reflecting actual job functions throughout your organization. Clinical trial managers receive appropriate EDC access, regulatory specialists access adverse event systems, quality engineers review complaint files, and executives monitor aggregate metrics—all with audit trails documenting every ePHI access. Multi-factor authentication prevents unauthorized access even when credentials are compromised.
24/7 Security Monitoring and Incident Response
Our Security Operations Center monitors your ePHI systems continuously, analyzing logs from databases, file servers, network devices, and endpoints. Behavioral analytics detect anomalous activities suggesting compromised credentials or insider threats. When security incidents occur, our incident response team coordinates containment, forensic analysis, regulatory notifications, and remediation—ensuring you meet breach reporting obligations while minimizing operational disruption.
Business Associate Agreement Management and Vendor Risk Assessment
We maintain centralized registers of all vendors accessing or potentially accessing PHI, track BAA execution status, and conduct annual vendor risk assessments. When engaging new CROs, cloud providers, or consultants, we verify they’ll execute appropriate BAAs before PHI exposure occurs. This proactive vendor management prevents common OCR violations from operating without compliant agreements.
HIPAA Workforce Training Programs
Our compliance training specialists deliver role-specific HIPAA education addressing actual job responsibilities. Clinical research personnel learn EDC security features and proper PHI handling during site monitoring. Quality engineers understand secure complaint investigation procedures. IT administrators receive privileged access management training. Annual refreshers keep the workforce current on evolving threats and updated policies while maintaining documented training records OCR auditors examine.
Integration with FDA Quality System Requirements
Technijian’s healthcare IT consultants understand ISO 13485 quality management systems and FDA regulations governing medical device manufacturing. We help clients leverage existing quality processes for HIPAA compliance:
Our document control procedures align HIPAA policy management with design history file practices. CAPA systems track security incidents alongside product quality issues. Management review processes incorporate HIPAA metrics. Risk management methodologies transfer directly between device safety and information security contexts. This integrated approach achieves dual compliance efficiently without maintaining parallel regulatory programs.
Cloud Platform Expertise for Clinical Research Systems
Modern clinical trials demand cloud-based EDC platforms enabling distributed research teams to collaborate securely. Technijian specializes in configuring HIPAA-compliant cloud environments on Microsoft Azure, Amazon Web Services, and healthcare-specific platforms:
We implement proper encryption at rest and in transit, configure role-based access controls, enable comprehensive audit logging, establish backup and disaster recovery procedures, negotiate business associate agreements with cloud providers, and validate compliance with FDA Part 11 electronic records requirements. Our cloud specialists ensure platforms provide usability researchers need while maintaining bulletproof security protecting participant PHI.
Proactive Compliance Documentation and Audit Readiness
Technijian maintains comprehensive compliance documentation satisfying OCR audit requirements:
Monthly reports summarize security monitoring activities, access pattern analyses, incident investigations, and remediation actions. Annual risk analyses update your documented risk landscape as operations evolve. Policy manuals receive yearly reviews ensuring procedures reflect current practices. Training records document all workforce education activities. Business associate agreements stay current as vendor relationships change.
If OCR selects your organization for audit or investigation, we provide immediate support organizing documentation, coordinating responses, and engaging specialized legal counsel. Our clients consistently resolve OCR inquiries through corrective action plans rather than enforcement actions because documentation demonstrates good-faith compliance efforts.
Disaster Recovery and Business Continuity for Mission-Critical Research Systems
Clinical trial timelines can’t accommodate extended database outages. Regulatory submission deadlines don’t wait for disaster recovery. Technijian implements robust backup and business continuity capabilities ensuring ePHI availability despite system failures, cyberattacks, or natural disasters:
Encrypted backups replicate to geographically dispersed data centers providing resilience against localized incidents. Quarterly disaster recovery testing validates restoration procedures work when needed. Documented recovery time objectives align with business impact analyses. Incident response playbooks detail exact procedures for ransomware containment, data restoration, and regulatory notifications.
Strategic Technology Partnership Supporting Growth
Beyond tactical HIPAA compliance, Technijian serves as your strategic technology advisor as your medical device company grows:
Planning clinical trial expansion supporting pivotal studies? We design scalable EDC infrastructure. Pursuing HITRUST certification for major hospital system contracts? We guide you through implementation and assessment processes. Acquiring companies with existing research databases? We conduct IT due diligence and integration planning. Expanding internationally requiring cross-border data transfer solutions? We implement appropriate safeguards addressing GDPR and local privacy regulations.
Our managed IT services grow with your business, scaling from startup support through IPO readiness and beyond.
Stop Risking $50,000 HIPAA Penalties—Partner with Orange County’s Medical Device IT Compliance Experts
HIPAA violations aren’t abstract regulatory concerns—they’re existential business threats that devastate unprepared medical device companies through massive penalties, reputational damage, lost hospital contracts, and intensified FDA scrutiny. The $50,000 mistake isn’t just the OCR settlement—it’s the clinical trial delays, partnership losses, and competitive disadvantages that follow compliance failures.
Irvine’s medical device corridor demands more than generic IT support. Your clinical research systems, quality databases, and regulatory platforms require specialized HIPAA-compliant infrastructure implemented by professionals who understand both healthcare compliance and medical device operations.
Technijian has protected Orange County medical device manufacturers for over two decades, delivering HIPAA-compliant managed IT services that satisfy OCR auditors, enable secure clinical collaboration, and support business growth throughout Irvine, Newport Beach, Orange, Santa Ana, and Southern California’s MedTech ecosystem.
Contact us today at (949)-379-8500 or visit technijian.com to schedule your complimentary HIPAA compliance assessment.
Discover how proper investment in HIPAA-compliant IT infrastructure costs far less than a single enforcement action—while delivering strategic advantages that strengthen your market position and accelerate product development timelines.
Your medical device company deserves IT partners who understand that protecting patient data isn’t just regulatory compliance—it’s fundamental to the innovation that improves lives worldwide.
Technijian – Orange County’s Trusted Healthcare IT Compliance Partner Since 2000
Serving Irvine, Newport Beach, Orange, Santa Ana, Costa Mesa, Tustin, Anaheim, Mission Viejo, Lake Forest, Huntington Beach, Fullerton, and all of Orange County and Southern California
(949)-379-8500 | technijian.com